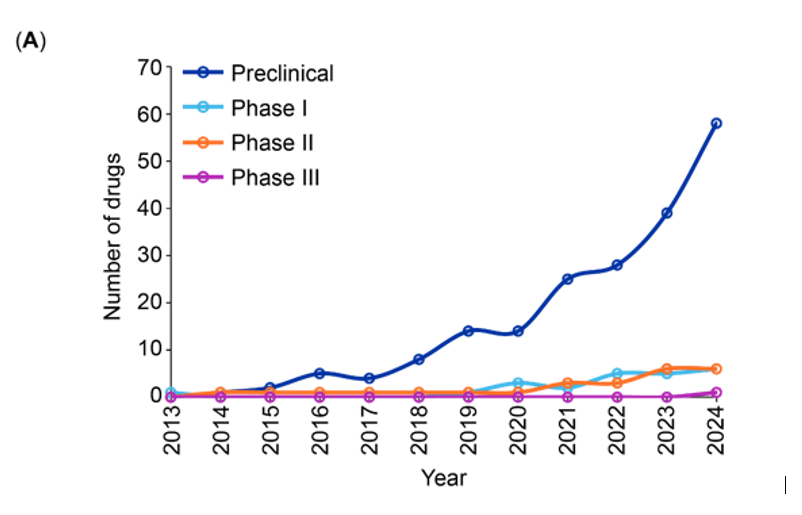
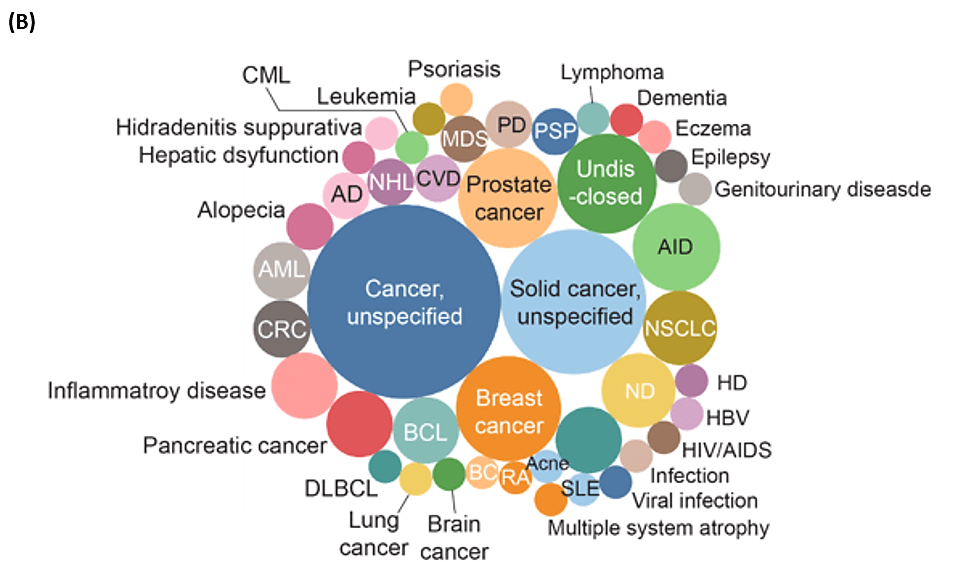
PROteolysis TArgeting Chimeras (PROTACs)- 2 decades of research
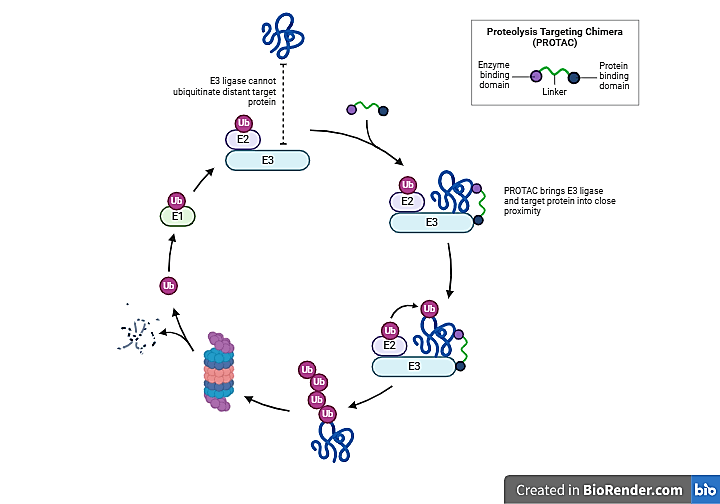
Researchers are investigating PROteolysis TArgeting Chimeras (PROTACs) as a potential treatment for cancer, neurodegenerative diseases, and other challenging conditions. PROTACs are small molecules that utilize the ubiquitin-proteasome system to degrade specific proteins. They function by linking the target protein to an E3 ligase, triggering its degradation while the PROTAC itself is recycled for repeated use.
Protein degradation is a natural process that removes damaged or misfolded proteins. PROTACs enhance this by linking target proteins, often linked to diseases like cancer, to E3 ligases. These ligases tag the proteins with ubiquitin, signalling the ubiquitin-proteasome system to degrade them. By facilitating this interaction, PROTACs promote targeted protein degradation. (Figure-1)
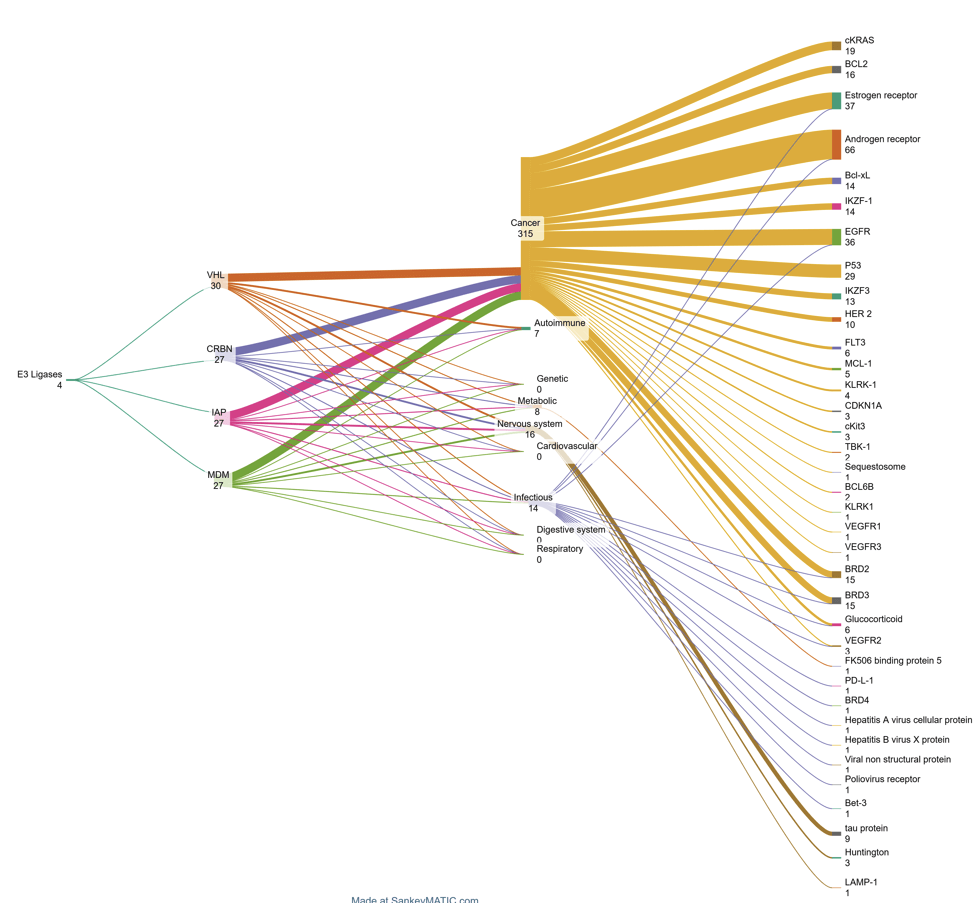
Figure 2. Sankey chart depicting E3 ligases (1st column), broad category of diseases (2nd column), disease targets (3rd column), and number of publications/patents (2003-2024; number against target)
Cancer is the primary focus of PROTAC research, followed by infectious and neurodegenerative diseases. Breast cancer, prostate cancer, and melanoma are the most studied, with recent advances in targeting ARs and ERs driving interest.
PROTACs target ARs, ERs, bromodomain proteins, and hard-to-treat proteins like KRAS and BCL2. While most progress is with AR and ER, ongoing research is expanding potential cancer treatments.
Preclinical trials are investigating PROTACs for proteins linked to Alzheimer’s, Parkinson’s, and Huntington’s, as well as kinases involved in HIV, hepatitis B, and autoimmune diseases. The expanding range of targets highlights PROTACs’ potential to treat challenging conditions.